Certification QSE
In 2016, PHARMACARE obtained certification according to ISO 9001:2008, ISO 14001:2004, and OHSAS 18001:2007 standards. A migration of this certification to the new 2015 versions of ISO 9001 and ISO 14001 standards took place in 2018, and a recertification of the integrated management system was carried out following a certification audit conducted in December 2021 according to international standards ISO 9001:2015, ISO 14001:2015, and ISO 45001:2018.
At Pharmacare, the meticulous application of Good Manufacturing Practices (GMP) is always the guarantee of minimizing the risk of cross-contamination, particularly through the implementation of operational procedures, risk analyses carried out for the introduction of new forms, products, and licenses, validation work on cleaning procedures, continuous monitoring of adherence to dress and hygiene rules in general, ongoing training and awareness programs involving all personnel, from management to operational staff, as well as the establishment of organizational measures and the display of strict instructions and guidelines to prevent contamination of any kind.
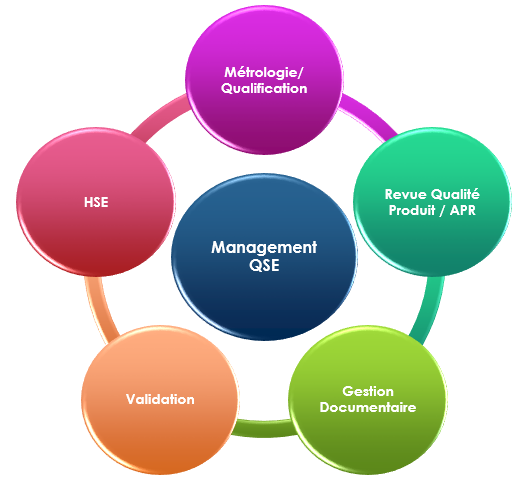
In this context, the QSE (Quality, Safety, and Environment) Management Department is involved in all activities of PHARMACARE Laboratories. It is responsible for setting quality standards and evaluating the effectiveness of the quality system. The QSE management ensures the company’s QSE Policy and consists mainly of:
- A unit responsible for metrology and equipment qualification
- A unit in charge of validation activities
- A unit responsible for product quality reviews before release and annual product quality reviews
- A unit responsible for document management
- A unit responsible for monitoring compliance with HSE (Health, Safety, and Environment) regulations